Continuously enhancing quality assurance and safety monitoring systems
To ensure that patients and healthcare providers are able to use our pharmaceuticals with peace of mind, it is crucial to monitor vigilantly their quality and safety even after their launch, and to take appropriate and prompt measures when necessary. The Law on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices requires strict controls to ensure quality and safety. Standards for control of pharmaceuticals and other products have been established, including Good Quality Practice (GQP) for quality and Good Vigilance Practice (GVP) for post-marketing safety.
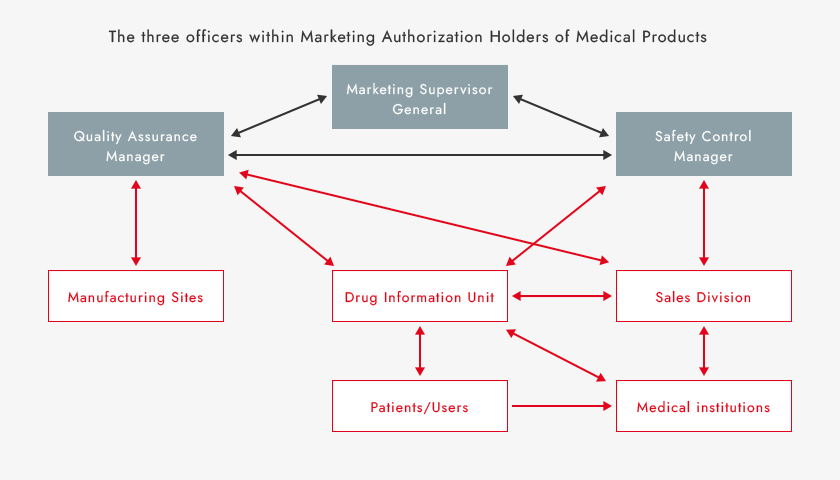
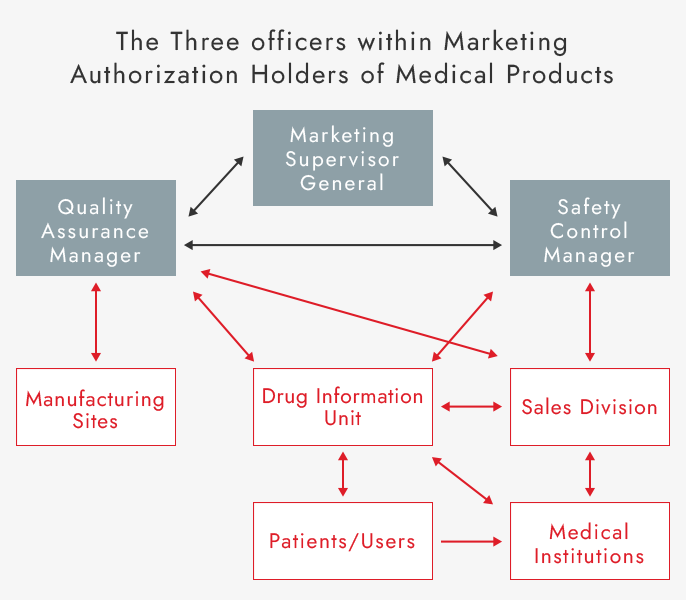
ASKA complies with these and other related laws, regulations and standards. As a pharmaceutical Marketing Authorization Holder, ASKA strives for close cooperation with our three key officers tasked with overseeing relevant activities and related departments. We are also enhancing employee training, strengthening our quality system and safety measures, and promoting proper use of pharmaceuticals. In addition, we collect and evaluate quality and safety information on our drugs from patients, medical institutions, and manufacturing sites, and take appropriate measures based on evaluations. By doing so, we conduct effective quality and safety control and work to continuously improve our pharmaceutical quality.
Quality Assurance and Safety Monitoring Systems and Information Flow