Overview

Based on our corporate philosophy to “Contribute toward the improvement of people's health and progress in medicine through the development of innovative products,” ASKA is enhancing its research and development capabilities to continuously create new high-value drugs that can address unmet medical needs. We have created many highly original products in-house utilizing our advanced technologies and accumulated years of experience in sex hormones and endocrinology, as well as with our dedication to open innovation. To further promote open innovation, we relocated our research center to the Shonan Health Innovation Park (Shonan iPark) in 2020. This has created a research system that can respond flexibly to rapid advances in technology and strengthened cooperation with external parties. Our development activities focus on the core areas of internal medicine, obstetrics and gynecology (Ob/Gyn), and urology. We are also working to develop products that will contribute strongly to health in niche areas and rare diseases. To conduct efficient and effective development, we carry out forward-looking alliance activities with partners in Japan and overseas with the aim of delivering useful new treatments to patients as quickly as possible.
Key business segments
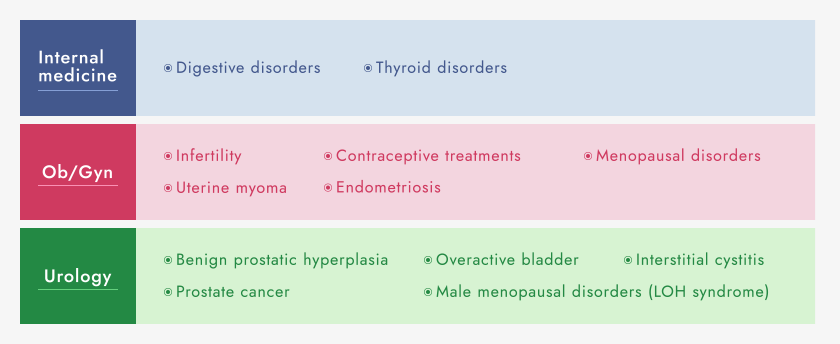
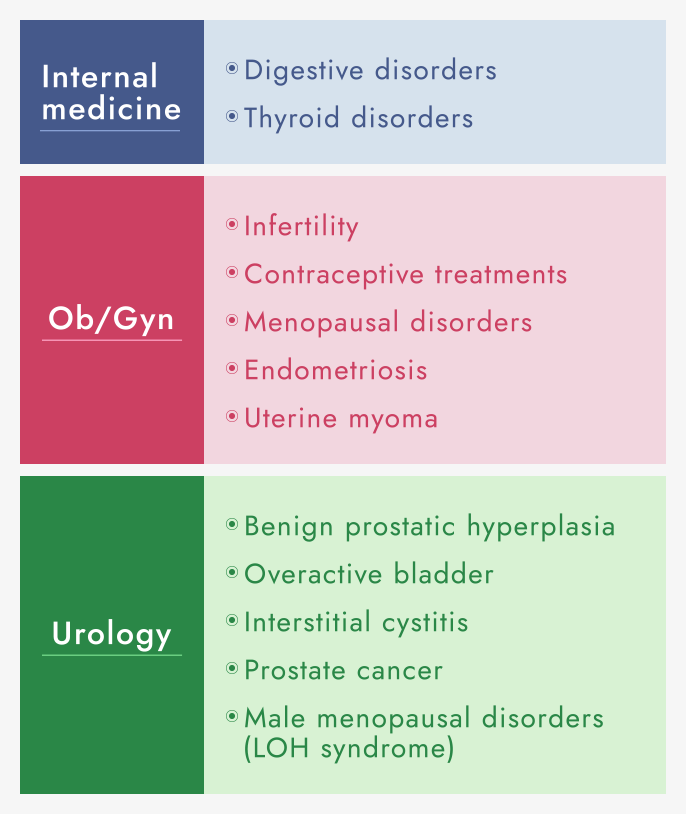
We are investigating the “seeds” of new pharmaceuticals, while focusing on our strengths in internal medicine (digestive organs and thyroid glands), Ob/Gyn, and urology. In addition to licensing out the products we have developed in-house, we are working on evaluating and developed in-licensed products. Aside from the treatment of diseases, we also investigate pharmacological “seeds,” alliances, and clinical development with an emphasis on total healthcare, from prevention to prognosis, with a view to improving quality of life.
Ethical considerations in research
ASKA has established internal regulations that comply with the Act on Welfare and Management of Animals and basic guidelines from the Ministry of Health, Labor and Welfare.*1 As well, the Company conducts ethical experiments using animals. All of our experiments are planned with the 3R*2 principles in mind and are examined by animal ethics committees, receive the approval of the director of the implementing organization, and are conducted in laboratories certified by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) respectively. We also perform internal inspections once a year. An ethical review committee has been set up to make judgments on using human tissue and on genetic research that confirm the appropriateness of such research.
- *1Basic guidelines for conducting experiments on animals, etc. at implementing organizations under the jurisdiction of the Ministry of Health, Labour and Welfare.
- *2The 3Rs refer to refinement (minimizing animal distress), replacement (using alternative methods), and reduction (reducing the number of animals used).
- *3A private nongovernmental organization that promotes the humane treatment of animals used in science through voluntary review and certification programs.

